Cancer Research / Clinical Trials
What are clinical trials?
Clinical trials are research studies in which patients may volunteer to participate. They are used to find better ways to prevent, diagnose and treat cancer. Clinical trials are part of a long, careful process, which may take many years. First, doctors study new treatments in the lab. Then they often study treatments on animals. If a new treatment shows promise then they test it on people. This is done in three or four phases.
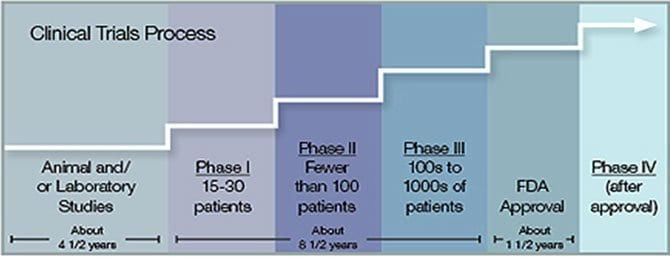
What is the clinical trial process or phases?
- Phase I Trials-Test if a new treatment is safe oin people. Doctors also find the best way to give the treatment.
- Phase II Trials -Test if a new treatment works in one type of cancer.
- Phase III Trials-Test if a new treatment is better than standard treatment.
- Phase IV Trials -Find more information about long-term side effects.
Am I able to take part?
 Not all clinical trials are right for all patients. A trial may be safe for one patient to join, but not safe for another. Each protocol has strict rules that doctors must follow to decide who may join the clinical trial. These rules are called eligibility criteria. This protects patients from getting treatment that may harm them.
Not all clinical trials are right for all patients. A trial may be safe for one patient to join, but not safe for another. Each protocol has strict rules that doctors must follow to decide who may join the clinical trial. These rules are called eligibility criteria. This protects patients from getting treatment that may harm them.
How do I sign up?
If your doctor offers you a clinical trial, you will first go through a process called informed consent. The goal of informed consent is to make sure you understand the clinical trials plan. The doctor or research nurse will review the informed consent form in detail with you. This form explains the clinical trials purpose, plan, risks and benefits. Take time to make your decision.
Who would be in charge of my care in the clinical trial?
Your doctor and nurse will still care for you. In a clinical trial, you will also have a:
- Principal Investigator (PI): The PI is usually a doctor. He or she runs the clinical trial and makes sure that the health care team follows the plan.
- Research Nurse: The research nurse teaches patients about the trial and collects data from patients on the trial. The research nurse is a good contact if you have questions during a clinical trial.
Would there be any follow-up after the clinical trial?
Yes, you would continue to see your doctor for treatment and follow-up care.
Would I be allowed to quit the clinical trial?
All patients in clinical trials are volunteers. You can choose to quit a clinical trial at any time, but talk to your doctor first. Your doctor can tell you how quitting the trial might affect your health and if there are other treatment options. Your relationship with your health care providers will not be changed by your decision.

“ Clinical trials are an important part of any quality cancer program. So many of our patients will be faced with life threatening diseases, for which there are few options and no cures. Having a clinical trial available to a patient with few proven options is so important for that patient and the patients with that disease that follow in the future. We would not have any effective treatments for cancer at all if it were not for clinical trials… ”
Linda Sutton MD,
Duke Oncology Network